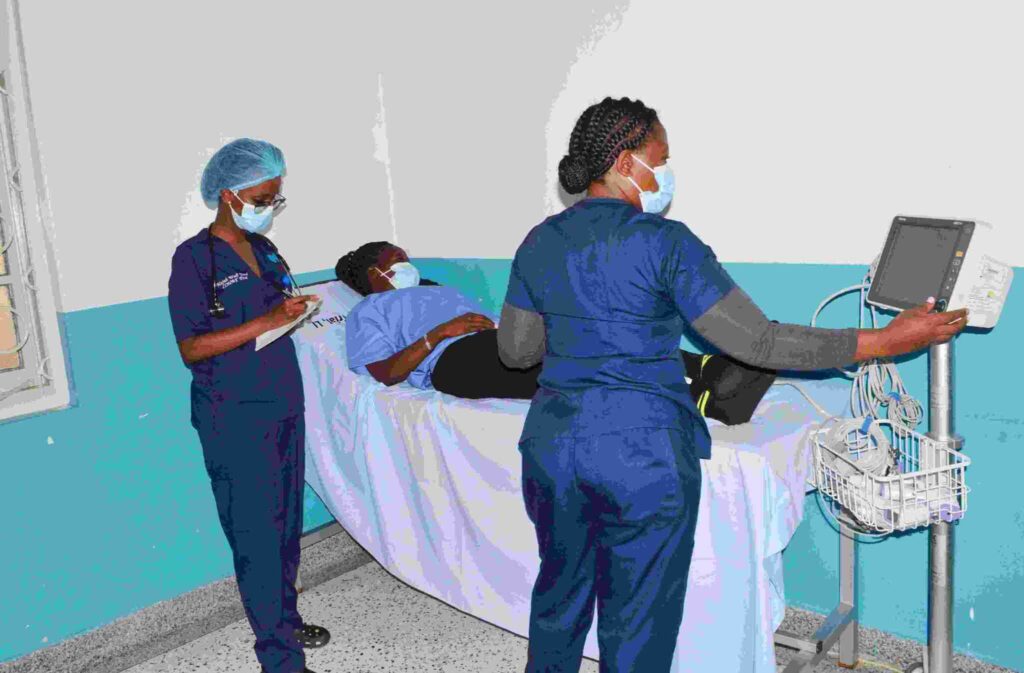
As Uganda joins the rest of the world in celebrating Clinical Trials Day today, Makerere University Lung Institute (MLI, is spearheading the Clinical Trial of Natural Therapeutics (CONAT) program. This ambitious endeavor, funded by the Ugandan government through the Science Technology and Innovation – Office of the President Secretariat (STI-OP), aims to uncover the healing powers of natural and herbal remedies, advancing them through human clinical trials to achieve notification and licensing of safe and efficacious products.
Clinical trials are essential for testing new treatments, including drugs and medical devices. In Uganda, a pioneering effort is underway to revolutionize healthcare through the CONAT program, which is not just about finding cures but also unlocking nature's potential.
According to Dr. Winters Muttamba, the CONAT Program Manager, they have successfully recruited their 510th trial participant in an ongoing trial focused on Acute Respiratory Tract Infections (ARVI) with romising results. This ARVI trial is assessing the safety, pharmacokinetics, and preliminary efficacy of Tazcov and Vidicine for treating acute respiratory viral infections, including SARS-CoV-2, Influenza A and B, and RSV.
This trial aims to evaluate Ugandan-formulated natural medicines for managing ARVIs, which are prevalent in the Ugandan population. While various local natural medicines exist, none have undergone formal scientific trials, causing the country to miss out on potentially effective treatmentsand their economic benefits.
The journey does not end with the CONAT program; we are extending our efforts to tuberculosis research, enhancing treatment outcomes, and reducing the disease burden," echoes Dr. Joanita Nalunjogi, the Clinical Trials Head at Makerere University Lung Institute.
Dr. Nalunjogi reveals that recent breakthroughs from the evaluation of a Standardized Treatment Regimen for patients with Multi-drug Resistant Tuberculosis (STREAM) trial may redefine the management of drug-resistant TB. This groundbreaking research confirmed that a shorter (6 or 9 months), safer regimen for drug-resistant TB is as effective as the traditional 12-month approach, offering a better outlook for patients and their caregivers.
All these breakthroughs are hinged on collaborative partnerships. The STREAM trial, a first of its kind in MDR-TB treatment evaluation, involved multiple research sites in Uganda, South Africa, India, Ethiopia, Moldova, and Georgia. Together, they aimed to identify a shorter, safer regimen for patients with MDR- TB and an all-oral regimen. Uganda contributed 56 out of 588 patients enrolled in the trial. Uniquely, the cost to Ugandan trial participants of the new 9-month treatment with pills was higher, as they spent more on additional food to supplement their diet during treatment, with no clear explanation.